Updated: 22/06/2021
The Malaysian Reserve reports that our government is spending RM3.5billion on vaccine procurement and logistics, which is an increase from last year’s estimated amount. According to the World Health Organisation (WHO), there are more than 150 potential vaccines that are currently being developed and tested globally to tackle the ongoing COVID-19 pandemic. Around 89 of these are in human trials (correct as of 3rd May 2021) and 27 are in the final stages of testing. Among them are a handful of vaccines that Malaysia is either looking to acquire or has already secured.
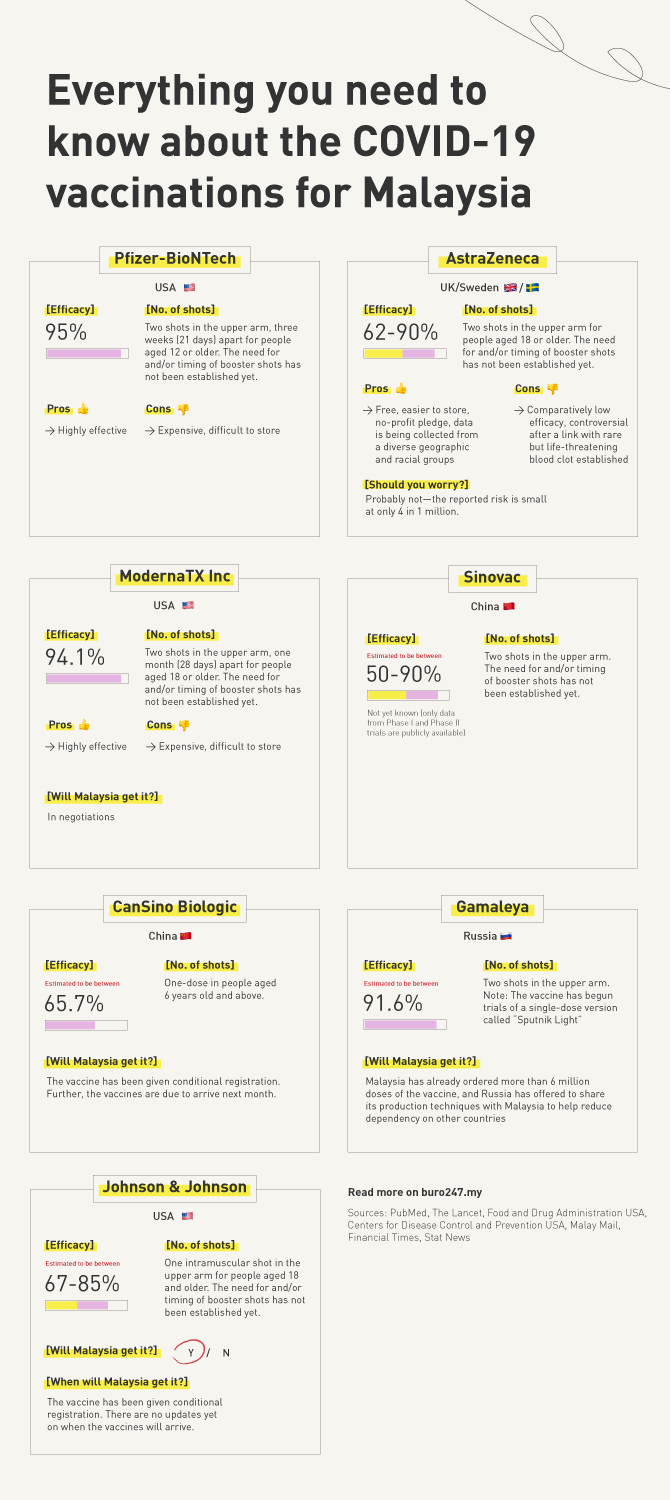
Ahead, find a break down of the most promising contenders so far—here are all the essential details behind each vaccine below (including the AstraZeneca-Oxford University vaccine).
Firstly, does efficacy actually matter?
The short answer is yes, but not in the way you think. While it seems easy enough to rank each vaccine by its efficacy, it isn’t always the most reliable indicator of a “good” vaccine. For starters, the data we have is entirely derived from clinical trials—these trials are conducted on a comparatively small scale, and therefore do not accurately represent the general population as a whole. Instead, they give an idea of what we can expect from the vaccine performance-wise and establish the safety of the vaccine for human inoculation.
Further, the efficacy numbers we have only give us an indication of how the vaccines performed in their individual trials. That’s why there is so much variation in some of the reported efficacies: A trial of Sinovac’s CoronaVac vaccine conducted in Brazil versus another trial of the vaccine conducted in Indonesia are bound to have widely different results. You have to take the different parameters and conditions into consideration, such as mutated viral strains and differing inclusion criteria, when looking at efficacy data.
Further, a vaccine’s efficacy measures its ability to prevent severe disease, hospitalisation, and death as a result of the disease. So far, all of the vaccines distributed have proven successful in preventing COVID-19-related hospitalisations and death, which means they are all performing as required.
With that in mind, here’s the TL;DR version summarising the need-to-know highlights of each main vaccine (scroll past for more details):
For a breakdown of the types of COVID-19 vaccines currently in development and their mechanisms of action, click here.
For those of you looking for a more in-depth summary, here are the details:
NOTE: The following vaccines are listed according to manufacturer and the country of origin—the name of each vaccine is listed under each heading.
Currently, Malaysia has approved 3 vaccines for use:
1. Pfizer-BioNTech, USA
Name: Trade-named Comirnaty, also known as Tozinameran (INN) and BNT162b2
Efficacy: 94-95% effective at preventing symptomatic COVID-19 infection according to preliminary Phase III results (peer-reviewed)
Number of shots: 2 intramuscular shots in the upper arm, three weeks (21 days) apart for people aged 12 or older. The need for and/or timing of booster shots has not been established yet.
Potential side effects: Pain at injection site, fatigue, headache, muscle pain, chills, joint pain, fever, injection site swelling, injection site redness, nausea, malaise (general discomfort), and lymphadenopathy (swollen lymph nodes).
Pros: Highly effective
Cons: Expensive, difficult to store
What is it: A vaccine that contains genetic material in the form of messenger RNA. It contains a small piece of the SARS-CoV-2 virus mRNA which instructs the cells in the body to make the virus’ distinctive “spike” protein without causing disease. The presence of this “spike” protein in the body then elicits an immune response against the SARS-CoV-2 virus. Phase III clinical trials are still ongoing for the vaccine and will continue through to 2022, meaning that the safety, efficacy, tolerability, and duration of the immunity of the vaccine are still being fully assessed.
For more information on the full ingredient list, side effects, and potential severe adverse events, click here.
2. Sinovac, China
Name: Trade-named CoronaVac
Efficacy: Not yet known (only data from Phase I and Phase II trials are publicly available—no peer-reviewed Phase III trial data yet). Estimated to be anywhere between 50-90% effective, based on trials in Brazil, Turkey and Indonesia.
Number of shots: Two intramuscular injections in the upper arm, with the second dose administered 14-28 days after the initial dose. The need for and/or timing of booster shots has not been established yet.
Potential side effects: Pain, swelling, itchiness, redness, induration, headache, fatigue, muscle pain, nausea, diarrhoea, joint pain, cough, chills, loss of appetite, runny or blocked nose, and abdominal pain. In less common or rarer cases, you may experience fever, tremors, flushing, swelling, dizziness, drowsiness, vomiting, hypersensitivity, abnormal skin, constipation, and hiccups. For the full list of side effects, click here.
What is it: An inactivated vaccine that uses killed virus parts to elicit the desired immune response. Phase III clinical trials are still ongoing for the vaccine, meaning that the safety, efficacy, tolerability, and duration of the immunity of the vaccine are still being fully assessed.
For more information on the full ingredient list, contraindications, side effects, and potential severe adverse events, click here.
3. AstraZeneca/Oxford University, UK/Sweden
Name: COVISHIELD, AZD1222 or ChAdOx1 nCoV-19
Efficacy: 62-90% effective at preventing symptomatic COVID-19 infection according to preliminary Phase III results (peer-reviewed).
Number of shots: 2 intramuscular shots in the upper arm for people aged 18 or older. The need for and/or timing of booster shots has not been established yet
Potential side effects: Injection-site pain, tenderness, warmth, redness, swelling, induration, itch, malaise, muscle ache, joint pain, fatigue, nausea, headache, chills, and blood clots. NOTE: Younger people and women more likely to experience side effects.
Pros: Free, easier to store (can be kept at refrigerator temperature for up to six months), AstraZeneca have signed a no-profit pledge, the data is being collected from a diverse range of geographic and racial groups.
Cons: Comparatively low efficacy. Further, the vaccine has become controversial after a link with an exceedingly rare but life-threatening blood clot was established (heparin-induced thrombocytopenia).
Should you worry? Probably not—according to Britain’s Medicines and Healthcare products Regulatory Agency, the reported risk is approximately 4 in 1 million while the risk of death is approximately one in 1 million. In a more recent analysis, the European Medicines Agency puts the risk at about 10 in 1 million. Therefore, the risks are still extremely rare. So, if you have signed up for the AstraZeneca vaccine, rest assured that its benefits still outweigh the risk of contracting COVID-19. However, be aware of the risks and associated symptoms following your vaccination.
For a rundown of the vaccine, the Malaysian government has put together a factsheet with the key information.
What is it: The vaccine is a modified chimpanzee adenovirus vector. This means that the vaccine is made from a cold virus that used to infect chimpanzees, but it has been modified to prevent infection in humans and carry the genetic “blueprints” for the COVID-19 “spike” protein instead. Once in the human body, the blueprints allow the body to produce the spike protein, which elicits the desired immune response.
For more information on the full ingredient list, contraindications, side effects, and potential severe adverse events, click here.
4. ModernaTX Inc, USA
Name: mRNA-1273 or CX-024414
Efficacy: 94.1% effective at preventing symptomatic COVID-19 infection according to preliminary Phase III results (peer-reviewed)
Number of shots: 2 intramuscular shots in the upper arm, one month (28 days) apart for people aged 18 or older. The need for and/or timing of booster shots has not been established yet
Potential side effects: Pain, swelling, and redness at the injection site, tiredness, headache, muscle pain, chills, joint pain, swollen lymph nodes in the same arm as the injection, nausea and vomiting, and fever. More people experienced side effects after the second dose than the first.
Pros: Effective
Cons: Expensive, difficult to store
What is it: Similar to Pfizer-BioNTech’s, it’s a vaccine that contains genetic material in the form of messenger RNA. It contains a small piece of the SARS-CoV-2 virus mRNA which instructs the cells in the body to make the virus’ distinctive “spike” protein without causing disease. The presence of this “spike” protein in the body then elicits an immune response against the SARS-CoV-2 virus. Phase III clinical trials are still ongoing for the vaccine, meaning that the safety, efficacy, tolerability, and duration of the immunity of the vaccine are still being fully assessed.
Will Malaysia get it?: In negotiations
For more information on the full ingredient list, side effects, and potential severe adverse events, click here.
5. CanSino Biologic, China
Name: Convidecia, also known as Ad5-nCoV
Efficacy: Estimated to be 65.7% effective, according to an interim analysis of Phase III trials (not peer-reviewed yet).
Number of shots: 1 dose in people aged 6 years old and above
Potential side effects: Not fully established yet
What is it: This vaccine is vector-based. It uses a genetically-engineered DNA sequence (called the adenovirus type 5 vector) to artificially carry the code for the SARS-CoV-2 “spike” protein into the body’s cells. This allows the body to produce the protein, which triggers the desired immune response. Phase III clinical trials are still ongoing for the vaccine, meaning that the safety, efficacy, tolerability, and duration of the immunity of the vaccine are still being fully assessed.
Will Malaysia get it?: According to The Star, the vaccine has been given conditional registration. Further, the Malay Mail reports that the vaccines are due to arrive next month.
6. Gamaleya, Russia
Name: Trade-named Sputnik V, also known as Gam-COVID-Vac
Efficacy: Estimated to be 91.6% effective, according to an interim analysis of a Phase III trial (peer-reviewed).
Number of shots: Two doses, with the second dose administered 21 days after the first dose. NOTE: The vaccine has begun trials of a single-dose version of the vaccine, called “Sputnik Light“.
Potential side effects: Flu-like illness, injection site reactions, headache, and weakness (not suly established yet).
What is it: This vaccine is vector-based. It works similarly to the former two vaccines; it uses both the non-replicating Adenovirus types 26 and 5 in separate doses as vectors to deliver the “spike” protein and cause the desired immune response. The first dose uses the Ad26 adenovirus, while the second dose uses the Ad5 adenovirus. This way, after the immune system creates antibodies against the first adenovirus, the second dose isn’t rendered ineffective. Phase III clinical trials are still ongoing for the vaccine, meaning that the safety, efficacy, tolerability, and duration of the immunity of the vaccine are still being fully assessed.
Will Malaysia get it?: According to the Malay Mail, Malaysia has already ordered more than 6 million doses of the vaccine, and Russia has offered to share its production techniques with Malaysia to help reduce dependency on other countries.
7. Johnson & Johnson, USA
Name: Trade named Janssen COVID-19 Vaccine, also known as Ad26.CoV2.S
Efficacy: 67% reduction in symptomatic COVID-19 disease and 85% effective at preventing COVID-19 related hospitalisation and death according to an interim analysis of Phase III results. No data on whether it prevents transmission yet.
Number of shots: One intramuscular shot in the upper arm for people aged 18 or older. The need for and/or timing of booster shots has not been established yet.
Potential side effects: Pain at injection site, injection site swelling, injection site redness, fatigue, headache, muscle pain, fever, and nausea. The risk of blood clots is extremely rare, but in the case of blood clotting, you may experience shortness of breath, chest pain, leg swelling, persistent abdominal pain, severe or persistent headaches or blurred vision, and easy bruising.
Pros: Easy to store (stable for two years at -25ºC to -15ºC, and a maximum of three months at 2ºC to 8ºC)
Cons: Comparatively low efficacy. This vaccine has also become controversial after a link with a very rare blood clot was found.
What is it: The Janssen vaccine is also a recombinant vector vaccine, and it uses a human adenovirus to express the SARS-CoV-2 spike protein. The adenovirus vector used in the experimental vaccine has been modified to prevent illness in humans, so it is safe.
Will Malaysia get it? Yes
When will Malaysia get this vaccine? The vaccine has been given conditional registration. There are no updates yet on when the vaccines will arrive.
Who should not get vaccinated:
- Those who have had severe or immediate allergic reactions to any ingredients in the vaccine
- Those who have had severe or immediate allergic reactions to the first dose of the vaccine
- Those who are pregnant or lactating
- Those on certain medications or underlying medical conditions
IMPORTANT: This is not a substitute for medical advice. Speak to your healthcare provider for your options.
What do we still not know?
- Whether the vaccines prevent the transmission of COVID-19 to others
- The long-term effects of each vaccine on the body
It must be noted that these vaccines have been approved for emergency use—considering the circumstances, it is expected for the vaccines to be distributed while still in Phase III. The research groups and regulatory bodies behind the available vaccines are confident that 1) the science behind the vaccines is robust, and 2) that the likelihood of severe adverse events occurring is low, and 3) that the benefits of the vaccines outweigh the risks. That said, as with any drug on the market (including common drugs like aspirin and paracetamol), there are bound to be side effects or adverse events that occur within some individuals.
So, at this point, healthcare providers and research groups are going to be monitoring the wider population for any new developments. It is, therefore, imperative that you are diligent in reporting any adverse reactions you may experience after taking the vaccine to your doctor. From there, they will be able to tr
| SHARE THE STORY | |
| Explore More |




